A Vinnova-funded (2023-2028) public–private collaboration aimed at demonstrating solutions that support the transition of healthcare from intervention-based care to continuous health management along the patient trajectory.
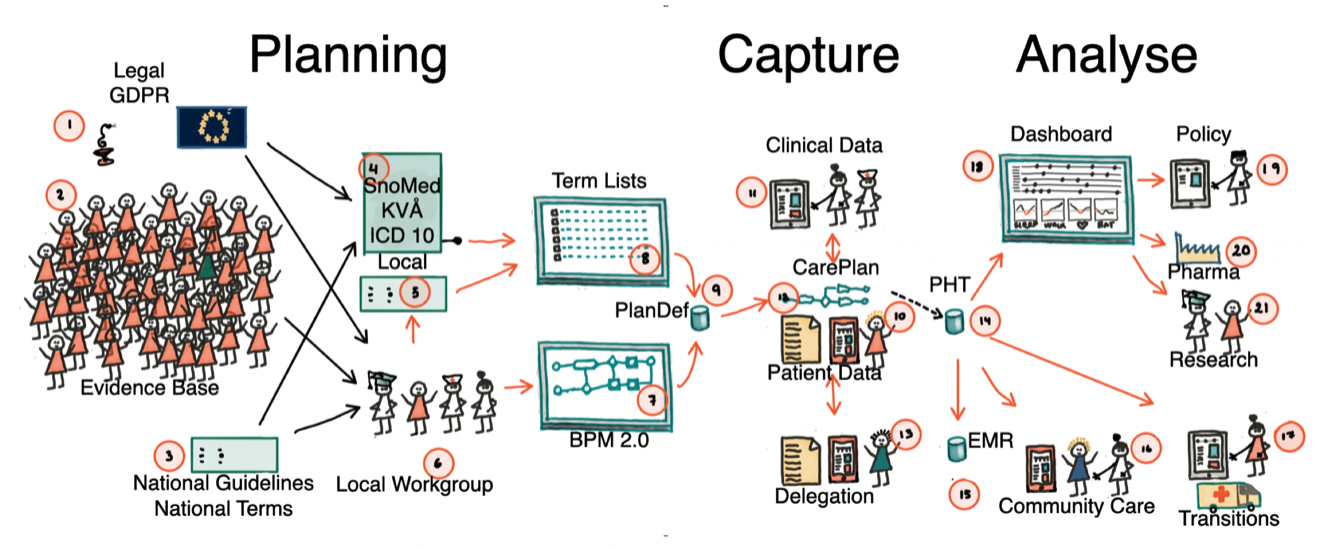
The project is based on preplanning data around locally developed healthcare processes. Once a workflow is defined, data collection can be fully structured and reused across EMRs, quality registries, and clinical research protocols. Better information service layer promotes interprofessional interoperability and patient participation.
Background
Semantic interoperability enables healthcare systems to share and understand data consistently, ensuring information keeps its meaning across settings. This is essential for outcomes-driven services, where accurate, comparable data is required to measure results, improve care quality, and drive evidence-based decisions. It is a foundation in the drive for personalized/precision medicine.
Subprojects
Subproject 1
Specialty level healthcare with concomitant processes for collecting the correct information for better clinical management, EMR documentation, reporting to quality registry, clinically embedded observational research. More than 800 patients have participated. Partners: Region Västra Götaland, Swedish Airway Quality Registry, Cambio, Karolinska Institutet.
Subproject 2
Distributed support for patients with rare diseases. The development of standard information models for support of self management in rare diseases gives an opportunity to develop assistance for correct management of the disease in question across national borders. A forceful application of known standards and principles of semantic interoperability simplifies the development of real-time support and quality control. Partners: SOBI/FLORI, Karolinska Institutet.
Subproject 3
Patient self-monitoring is highly desired by patients and providers. The current legislation and technical shortcomings of available information systems has resulted in a wide spread development of monoliths. While being excellent within diagnosis and data-stream they are becoming expensive and difficult to maintain from healthcare. The project aims at understanding the general requirements and real world test of the developed theories. Partners: Region Uppsala, Medituner AB, Cambio, Astma Allergi Förbundet.
Subproject 4
Secondary prevention in heart disease could possibly diminish further morbidity and improve quality of life. The ability to follow patients following the first year is low due to care structure and the low priority for immediate action in primary care. We have developed a simple administrative routine for calculating the need to meet the patient based on quality registry data. We now aim to connect this routine to secondary prevention support in primary health care. Partners: Region Uppsala, Karolinska Institutet, Novartis. Other partners will join.
Highlighted Principles
Common to all activities are the following:
Local Planning Tools
Planning of care must be done with locally available tools
Real-time Principles
Preplanning of data allows for real time principles in feedback
Avoid Post-organisation
Avoid post-organisation of data due to costs and risk of errors
Standards Compatibility
Always be prepared for Open EHR, FHIR and OMOP compatibility









